Reading Apprenticeship Inspired Assignment or Lesson
Reading Apprenticeship Inspired Assignment or Lesson
Students often struggle with pH and pKa in chemistry because these concepts involve a complex interplay between logarithmic scales, the understanding of acid-base equilibria, the ability to interpret molecular structure to estimate pKa values, and often require a solid grasp of basic math skills, which can be challenging for many learners, especially when applying them to real-world chemical scenarios; additionally, the abstract nature of these concepts, where students need to visualize the movement of protons at the molecular level, can lead to confusion. A “Think Aloud” is a strategy that helps students make sense of their thinking.
Chemical information literacy, particularly regarding pH and logarithmic relationships, is an essential skillset for navigating, evaluating, and using the wealth of print and online chemical data. A “Think Aloud” will improve students' acquisition and mastery of logarithmic reasoning and pH calculations in chemistry. In addition, metacognition associated with a text will assist students' in finding and applying logarithmic information to solve complex acid-base problems, especially when pH calculations are involved.
This activity focuses students' abilities in one critical area of chemical information literacy: finding, estimating, and using pKa values to calculate pH in organic acid-base problems. This includes understanding the logarithmic relationship between [H+], pH, and pKa, as well as applying the Henderson-Hasselbalch equation to buffer systems. After years of teaching organic chemistry, I have identified areas of student difficulty related to these skills, such as confusion about the inverse logarithmic relationship between pH and [H+], challenges with significant figures in logarithmic calculations, and difficulties understanding how pKa relates to acid strength. I then designed a text and implemented a “Think Aloud” with students aligned with desired learning outcomes, emphasizing both the mathematical foundations of logarithms and their practical application in pH calculations. This lesson typically occurs in the first six weeks of the semester. It leverages students' prior knowledge about pH from General Chemistry I and pKa from General Chemistry II.
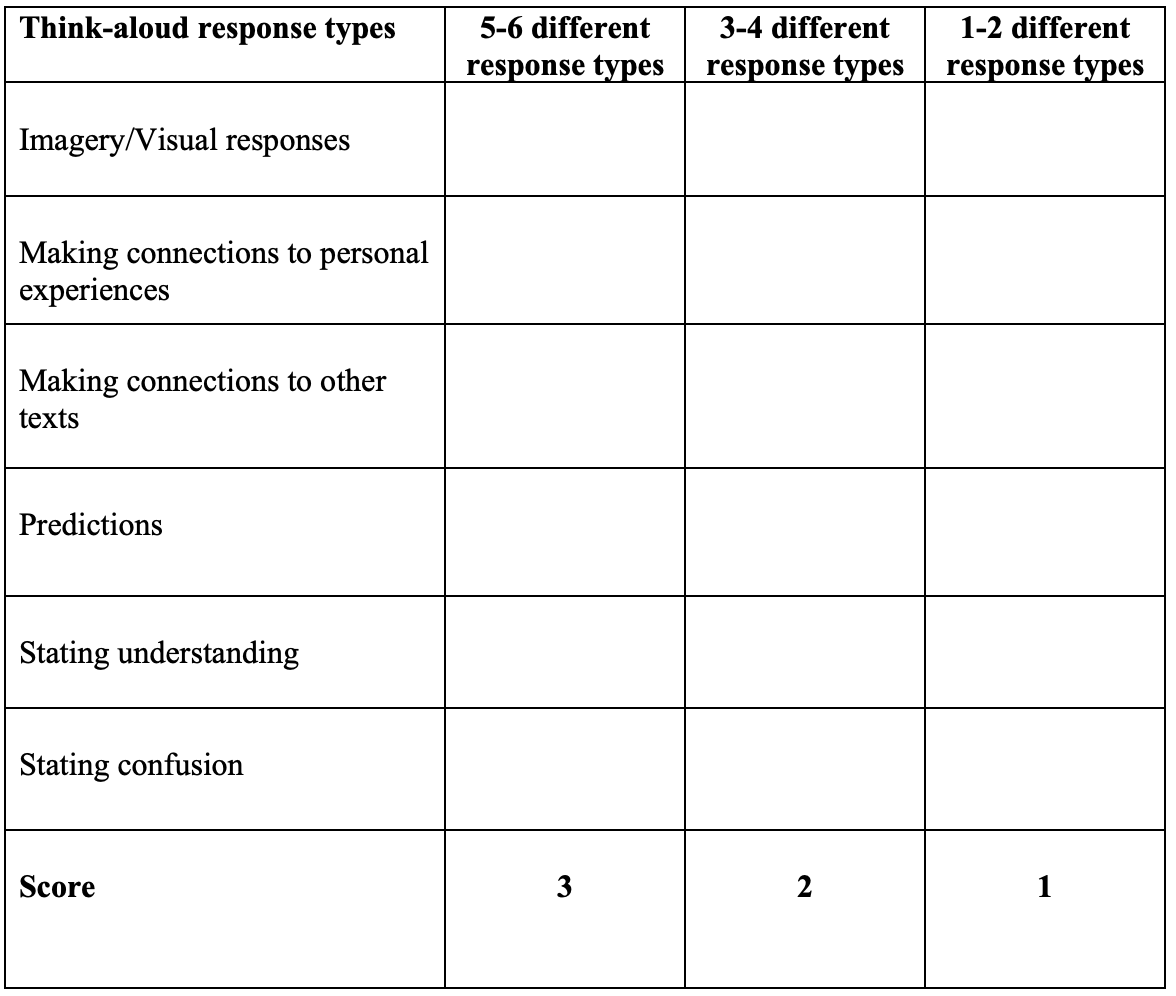
Reasons for difficulty with pH and pKa can include:
Logarithmic scale: The use of logarithms to express pH and pKa can be confusing for students who are not comfortable with logarithmic calculations or interpreting their meaning.
Conceptual understanding: Differentiating between pH (a measure of a solution's acidity) and pKa (a property of a specific molecule) can be challenging, especially when trying to apply them in different situations.
Molecular structure interpretation: Estimating pKa values often requires analyzing a molecule's functional groups and their influence on proton donation, which can be difficult for students who are still developing their understanding of organic chemistry.
Application to real-world problems: Applying pH and pKa concepts to complex chemical reactions or biological systems can be challenging, as it requires considering multiple factors like concentration, temperature, and the presence of other molecules.
Lack of visual representation: The abstract nature of proton transfer can be difficult to visualize, making it challenging to fully grasp the concept of acid-base equilibrium.
However, metacognition can address these challenges:
By emphasizing the basic principles of acids and bases, including proton donation and acceptance, before introducing pH and pKa calculations, a think aloud can encourage students to reflect on their prior knowledge by asking questions like "How do you visualize proton transfer?" and "What helped you understand Brønsted-Lowry acid-base pairs?" This metacognitive approach helps students build conscious connections between their existing understanding and new concepts.
When we use real-world examples and applications to illustrate how pH and pKa are important in various fields like biology, medicine, and environmental science, we can use metacognition to have students monitor their comprehension by explaining in their own words how concepts like blood pH buffering or ocean acidification work. When we ask them to identify which aspects of these applications make sense to them and which remain unclear, this helps them develop self-awareness about their own understanding.
I utilize diagrams, animations, and interactive simulations to help students visualize the molecular processes involved in acid-base reactions. However, I know that giving students time to “Think Aloud” and engage with text will guide students to think about their learning preferences. By asking "Which visualization method helped you most understand proton transfer?" or "How did the animation change your thinking about buffer systems?", this metacognitive reflection helps students identify effective learning strategies for abstract molecular concepts.
In order to ensure students are comfortable with basic logarithmic calculations and can interpret their meaning by having them articulate their problem-solving process, I ask questions like "What steps do you take when converting between [H+] and pH?" or "How do you check if your answer makes sense?" This self-monitoring helps students identify calculation errors and develop stronger mathematical reasoning skills.
When we introduce complex concepts like the Henderson-Hasselbalch equation, a “Think Aloud” is perfect for building procedural or step-by-step processes, building upon foundational knowledge. When students regularly pause to assess their understanding with prompts like "How does this equation connect to what you know about acid strength?" or "What aspects of buffer calculations are still challenging for you?", this metacognitive practice helps students identify knowledge gaps and develop strategies for mastering challenging calculations.
Students use a "Think Aloud" to engage with the following text: Have you ever wondered why increasing pH or pKa value indicates a lower concentration of acid in a solution (pH) or decreased ability to donate H+ (pKa)? It may be that you need to revisit a few mathematical principles to gain an understanding of how log makes it easier to determine the two situations described above.
Let’s start with a few definitions:
B/L acid: a compound which donates H+.
B/L base: a compound which accepts H+.
pH = -log [H+] where [H+] indicates a concentration in M or mol/L.
Note: these concentrations are usually less than 1.0M for safety reasons.
pKa = -log Ka where Ka is the dissociation constant for a specified weak acid.
Why do we use the -log?
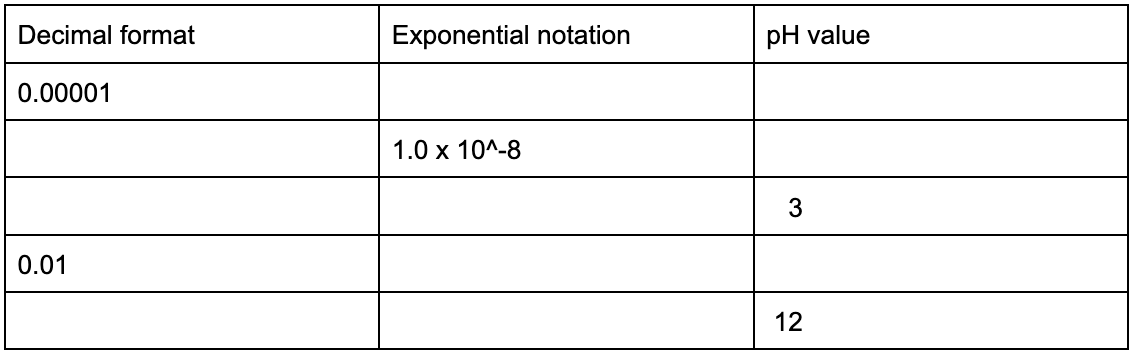
The function of log is shorthand notation for log base 10, which means that very large and very small numbers can be simplified to whole numbers by exponential notation in the form of 10^x. When a value between 0 and 1 is expressed, it can be further simplified by using the -log of the value. When a value greater than 1 and is very large is expressed, the same can be accomplished using positive log. What you will notice is that the first value of a logarithmic outcome expresses how many places the decimal point moved to arrive at the first non-zero value.
To help you get refamiliarized with values that can be expressed in different ways, please fill in the missing data below. Assume each value represents a molarity, mol/L, of H+ ions.
The following texts were used to develop the primary text for this activity: https://openstax.org/books/chemistry-2e/pages/14-2-ph-and-poh
https://openstax.org/books/organic-chemistry/pages/2-9-predicting-acid-base-reactions-from-pka-values
https://openstax.org/books/chemistry-2e/pages/14-3-relative-strengths-of-acids-and-bases


